主族金屬通常只有一種氧化態,而過渡金屬元素則常以多種氧化態存在於化合物中。下表列舉出第四週期的過渡金屬的常見氧化態。
| 過渡金屬離子 | 常見氧化態 | 化合物舉例 |
|---|---|---|
| \(\ce{Sc}\) | \(+3\) | \(\ce{Sc2O3}\) |
| \(\ce{Ti}\) | \(+2\),\(+3\),\(+4\) | \(\ce{TiO}\),\(\ce{Ti2O3}\),\(\ce{TiO2}\) |
| \(\ce{V}\) | \(+2\),\(+3\),\(+4\),\(+5\) | \(\ce{VO}\),\(\ce{V2O3}\),\(\ce{VO2}\),\(\ce{V2O5}\) |
| \(\ce{Cr}\) | \(+2\),\(+3\),\(+6\) | \(\ce{CrO}\),\(\ce{Cr2O3}\),\(\ce{CrO3}\) |
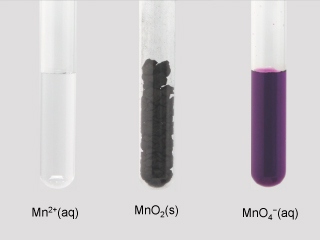
| \(\ce{Mn}\) | \(+2\),\(+3\),\(+4\),\(+6\),\(+7\) | \(\ce{MnO}\),\(\ce{Mn2O3}\),\(\ce{MnO2}\), \(\ce{K2MnO4}\),\(\ce{KMnO4}\) |
| \(\ce{Fe}\) | \(+2\),\(+3\) | \(\ce{FeCl2}\),\(\ce{FeCl3}\) |
| \(\ce{Co}\) | \(+2\),\(+3\) | \(\ce{CoO}\),\(\ce{Co2O3}\) |
| \(\ce{Ni}\) | \(+2\),\(+3\) | \(\ce{NiO}\),\(\ce{Ni2O3}\) |
| \(\ce{Cu}\) | \(+1\),\(+2\) | \(\ce{Cu2O}\),\(\ce{CuO}\) |
| \(\ce{Zn}\) | \(+2\) | \(\ce{ZnO}\) |
若過渡金屬處於較高氧化態,則通常是良好的氧化劑,如 \(\ce{Cr2O7^{2−}} \) 和 \(\ce{MnO4^{−}}\) 等;相反地,若過渡金屬處於低氧化態,則通常是良好的還原劑,如 \(\ce{V^2+}\) 和 \(\ce{Cr^2+}\) 等。